九强新闻
BSBE 2020 IFCC-RELA Reference Measurement Capability Verification Activity Result Announcement
Published:2020-10-28
Source:[英文]九强生物
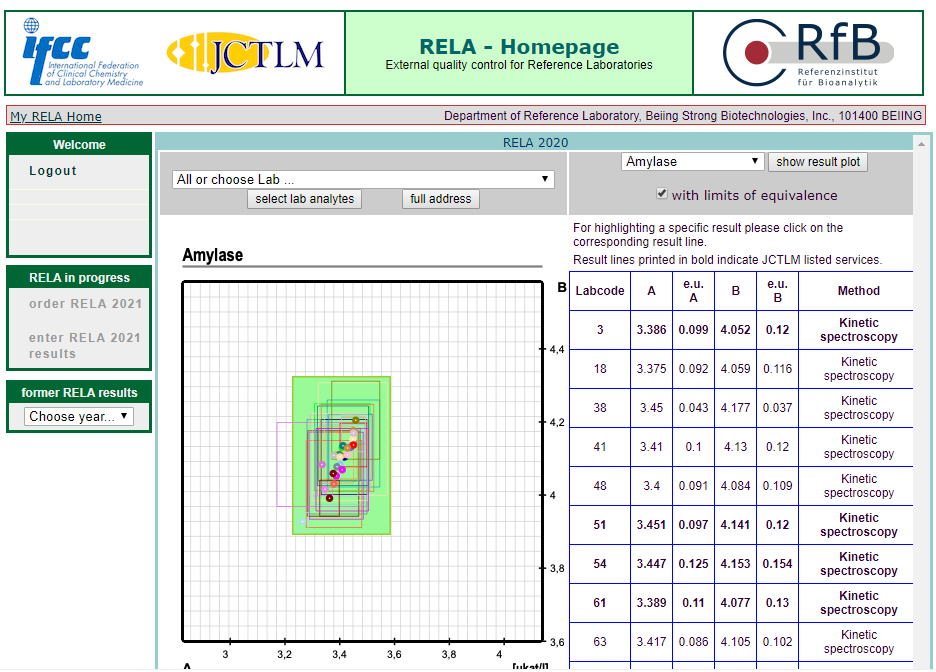
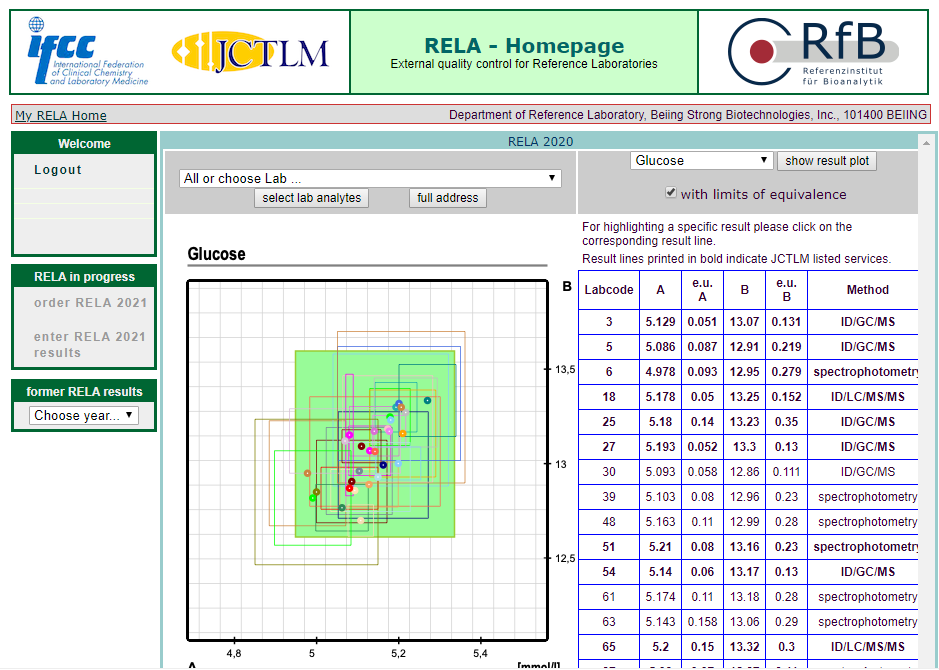
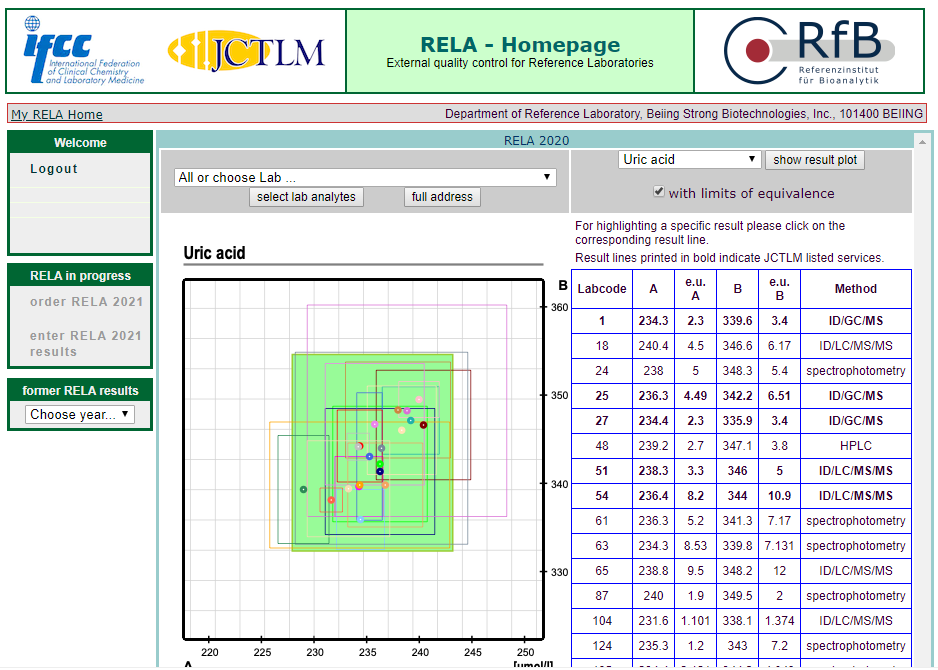
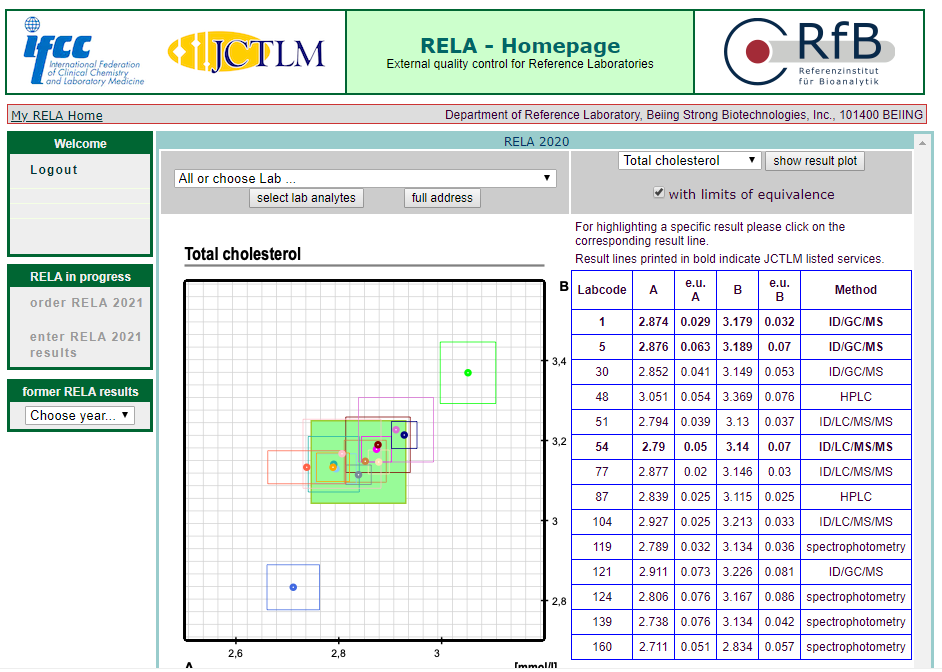
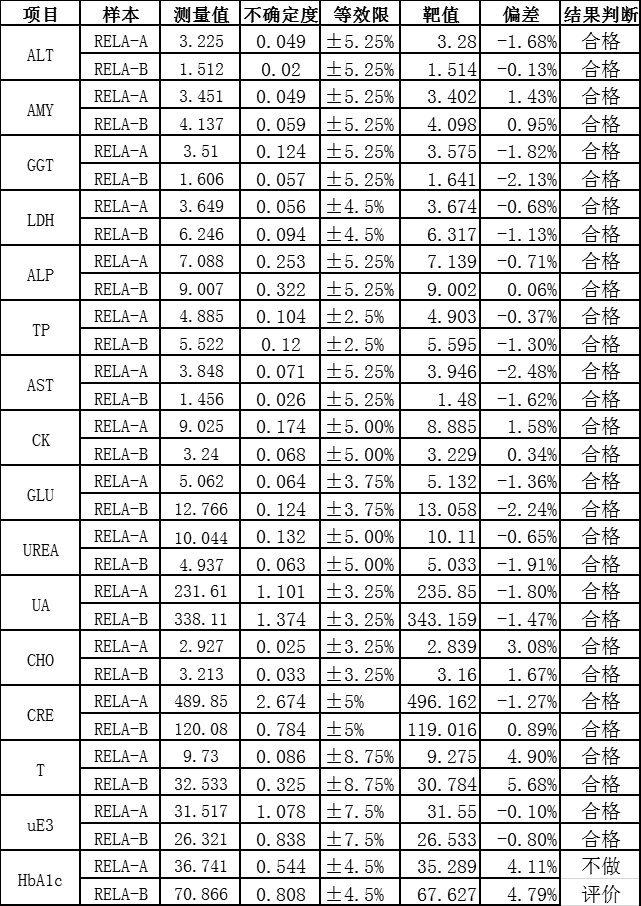
The results of 16 projects in rela 2020 were all qualified, including HbA1c, Glu, urea, TP, CRE, UA, CHO, alt, AST, Amy, ALP, CK, LDH, GGT, estriol, testosterone, involving enzymes, metabolites, protein, hormone, glycosylated hemoglobin and other projects.
According to ISO17025 and iso15195, the quality management system of the reference laboratory of the BSBE has been strictly operated, and has been approved by CNAs medical reference laboratory in 2018. At the same time, according to iso17511, a nine strong traceability system was established to trace the test results of in vitro diagnostic products to international standards, so as to ensure the accuracy of the test results of clinical samples.
The BSBE has been working hard to achieve the standardization and consistency of test results, actively cooperating with the Chinese Academy of metrology, the Chinese Academy of inspection and other institutions to carry out joint assignment of reference materials, participating in the formulation of industry standards and the compilation of expert consensus, and contributing to the development of the industry.